GMP: Annex 1 guidelines on dressing in cleanrooms
- Category: News
By the summer of 2023, all manufacturers of sterile healthcare products (active substances, excipients, primary packaging materials and finished galenic forms) will have to comply with the new recommendations in Annex 1 of the GMP (Good Manufacturing Practices).
This revision of Annex 1 makes Quality Risk Management the pivotal element in the control of contamination, whether microbiological, particulate or chemical.
As part of the process of dressing in cleanrooms, clothing and other personal equipment must be designed and now qualified in accordance with the guidelines defined by GMP. This initial qualification, supplemented by periodic requalification covering at least the lifespan of the said equipment, forms part of the definition of the contamination control strategy (CCS) and is a major element of it.
Cleanroom garments must therefore be selected with the utmost rigour, based on verified performance data, always with the aim of reducing the risk of contamination.
Filtration performance of single-use garments
The garment is the main barrier between the process/product pair and the primary source of contamination, i.e. the operator. The design of the garment and the materials used must guarantee the best possible filtration of biological and particulate contaminants.
The ability to effectively filter particulate and biological contaminants is the decisive criterion in the selection of a garment. Annex 1 tells us that no CFU (Colony Forming Unit) is accepted in a Grade A environment and only 5 CFU in a Grade B. This requirement means that operators must be equipped with garments capable of guaranteeing the highest performance.
There are currently two solutions for equipping an operator in an aseptic room: single-use garments and reprocessable garments. In recent years, several publications have highlighted the difference in contaminant filtration performance between single-use and reprocessable garments.
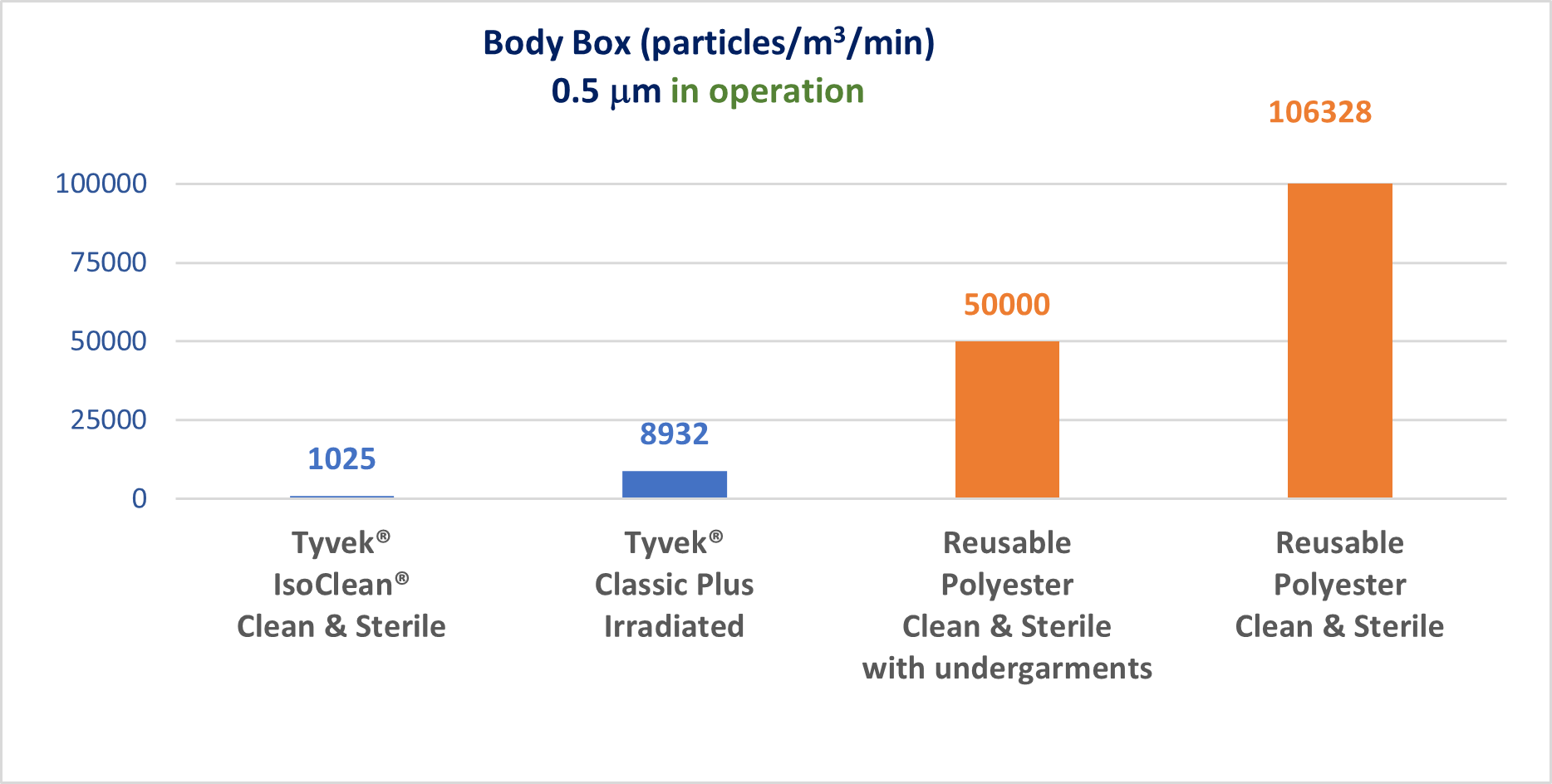 C. Moschner, Contamination Source “Human” or how efficient is Cleanroom Garment,
C. Moschner, Contamination Source “Human” or how efficient is Cleanroom Garment,
2017 & G. Maik, Work With Cytotoxic Drugs In Pharmacies & Pharmaceutical Industry, 2018
In addition to good filtration performance, the garment must not be a carrier of particulate or chemical contaminants. At DuPont™, the Tyvek® IsoClean® Clean & Sterile range offers garments and accessories that have first been washed in deionised water to remove particulate and chemical contaminants. They are then folded and packaged in an ISO 4 cleanroom, taking into account the specific dressing constraints of the operators, thus reducing the risk of contamination of the garment by them. Finally, the gamma-irradiation stage guarantees a level of sterility assurance equivalent to a logarithmic reduction of 10-6 according to ISO standard 11137.
Qualification and routine checks
Clothing and its quality must be adapted to the process and the requirements of the work area. They must be worn in such a way as to protect the product and its environment from contamination. Sometimes it is also necessary to protect the operator from the risks generated by the manufacturing or biocleaning process. When this is the case, the garment must not compromise the protection of the product against contamination. The single-use garment allows this protection of the operator thanks to its PPE claim, most often type 5 and 6. Protection claim not applicable to removable garments.
Compliance with aseptic dressing procedures and their performance must be confirmed by an initial assessment and regular routine reassessment. This involves a visual assessment of the cleanliness and integrity of the garment. Garment qualification should take into account all necessary garment testing requirements, including damage to garments that may not be identified by simple visual inspection. Reusable garments must be replaced if damage is identified and can no longer be repaired as was often the case in the past. This requires increased monitoring of service providers and the services they are able to provide or not.
In addition to visual inspection, a microbial assessment must be carried out regularly on monitoring areas such as gloves, forearms, chest or bonnet. A rigorous dressing procedure and the choice of high-performance equipment are key to maintaining asepsis.
And in non-aseptic areas?
The purpose of Annex 1 is to provide guidance in the manufacture of sterile products. However, certain principles, such as the contamination control strategy (CCS) and the qualification and validation of packaging, can be used for the manufacture of non-sterile products. This is explicitly mentioned in the new text. Products such as liquids, creams, biological intermediates and solid forms also require control and reduction of levels of particulate, chemical, endotoxic and bioburden contamination.
Cleanroom cladding and environmental impact
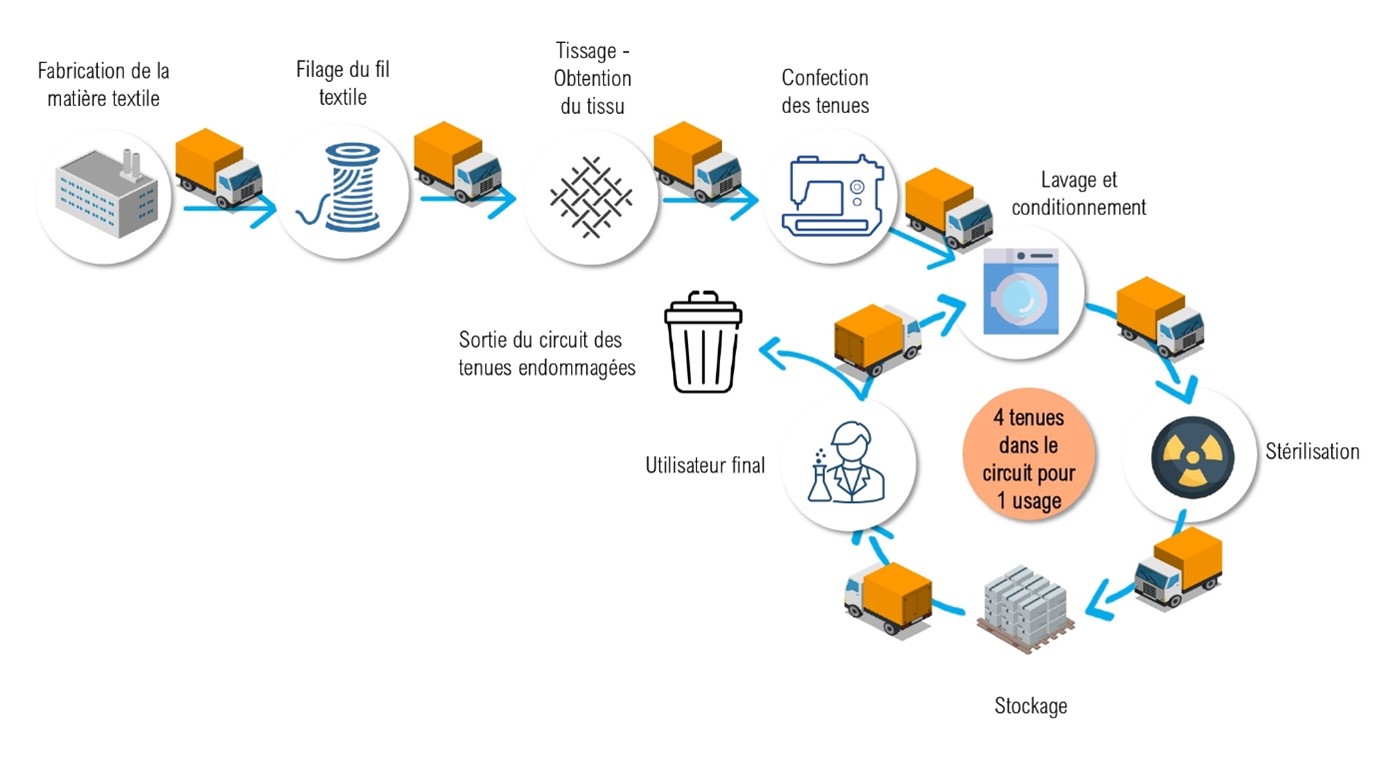 Production and reprocessing workflow for reusable cleanroom garments.
Production and reprocessing workflow for reusable cleanroom garments.Still in the case of disposable garments: is the impact of laundry products measured, as well as the energy expenditure linked to high temperature washing water and energy-intensive drying?
Aware of the environmental challenges, Conformat and its partners are committed to offering ever more responsible solutions, including the implementation of recycling solutions for disposable clothing. Its teams of experts are aware of these issues and provide support in qualifying products that are increasingly relevant to activities in controlled environments.




